Investigation of Transdermal Buprenorphine Patch for Use on Horses
Transdermal administration of buprenorphine via a patch has been investigated in animals such as dogs, cats, pigs, sheep, and primates. Paranjape VV. et al to their knowledge are the first to describe the use on horses. This study monitored the physiological conditions and pharmacokinetic profile of six horses under three different rounds of treatments. BUP0 was the first control treatment where horses did not receive a patch. The second (BUP20) and third treatments (BUP40) administered buprenorphine patches, resulting in doses of 0.03 µg kg-1 h-1 and 0.07 µg kg-1 h-1 respectively.
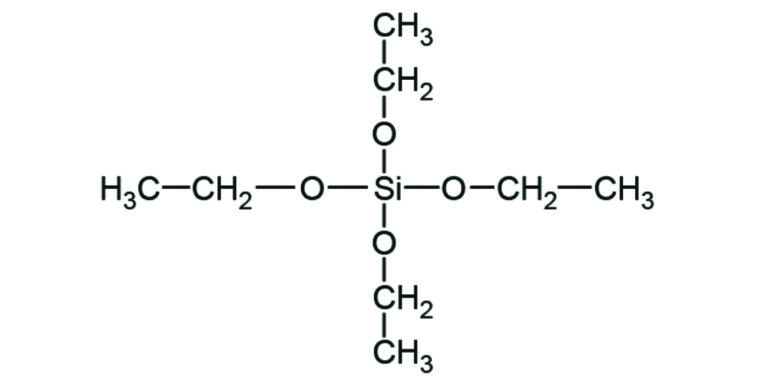
UCT’s Clean-Up C18 (unendcapped) 200 mg, 3 mL SPE column was used to extract buprenorphine from plasma samples. Plasma concentrations from samples were then used to calculate the pharmacokinetic profiles from treatments BUP20 and BUP40. 0.5 mL of sample was diluted with 2 mL of 0.1 M pH 6 phosphate buffer and spiked with internal standard. SPE columns were conditioned with 2.5 mL of methanol and 3 mL of water. After loading the sample, the column was washed with 2 mL of 50% methanol before eluting with 2.5 mL of methanol. Samples were evaporated and reconstituted before analysis on a LC-MS/MS. Time of maximum measured plasma concentration (Tmax) for BUP20 was 12.70 ± 3.93 h and for BUP40 was 11.33 ± 4.68 h. Maximum measured plasma concentration (Cmax) for BUP20 was 0.10 ± 0.04 ng/mL and for BUP40 was 0.21 ± 0.06 ng/mL.
This study suggests that transdermal administration of buprenorphine via a patch is a viable alternative to injection or oral administration. The two doses investigated in the study were tolerated well by the horses supported by the lack of changes in the physiological conditions of the horse. The correlation between plasma concentrations and increased thermal threshold by the horses shows the anti-nociceptive effect of the drug on the horses. However, the authors recognize this study has limits such as a small sample size (n=6) and the absence of behavioral analysis. Further research is still needed.
Citation: Paranjape VV, Knych HK, Berghaus LJ, Cathcart J, Giancola S, Craig H, James C, Saksena S, Reed RA. Evaluation of physical variables, thermal nociceptive threshold testing and pharmacokinetics during placement of transdermal buprenorphine matrix-type patch in healthy adult horses. Front Pain Res (Lausanne). 2024 Mar 11;5:1373555. doi: 10.3389/fpain.2024.1373555. PMID: 38529072; PMCID: PMC10961409.